Time to Open-Source Ventilators
Thus far, the U.S. government has yet to come up with a plan to produce the amount of ventilators that experts say are necessary to serve patients afflicted with COVID-19. It should now force ventilator makers to make their designs open-source.
By experts and staff
- Published
By
- Robert K. KnakeWhitney Shepardson Senior Fellow
A month into this crisis, and government and private industry have yet to foment a plan to produce the number of ventilators that experts say are necessary to get us through the coronavirus pandemic. Absent a massive ramping of production, doctors will be forced to ration the equipment, making heartbreaking decisions about who will live and who will die.
Efforts to let the private sector recognize the “opportunity” created by the crisis are unlikely to produce the equipment necessary in time. Manufacturers have asked the government to provide certainty for that opportunity by extending contracts to guarantee production runs so that they can make decisions about how to ramp up production.
Providing certainty to existing manufacturers is only one part of the solution. The other is to dramatically expand the production base. The president should use his authority under the Defense Production Act to force makers of ventilators in the United States to make the design specifications for their equipment publicly available so that other manufacturers can readily identify what capacities they have to produce the equipment or subcomponents of it.
There is some precedent for the federal government taking this action. In his book After on the American response to 9/11, Steven Brill recounts how the Transportation Security Administration forced InVision, then the lone maker of advanced explosives detection equipment, to provide the government all its intellectual property so that other manufacturers could produce it.
The deal gave InVision a fee equal to 8 percent of the list price for each unit produced by another manufacturer (a major hit to InVision, which typically made 30 to 40 percent on each unit it produced). The government’s rights lasted for two years and, crucially, did not include the company’s newest and best models. The contract for this deal from 2002 is available on the SEC website here.
In the case of the explosives detectors, the government had a security interest in not releasing the design plans to the general public. In the case of ventilators, and other needed equipment like personal protective equipment, the interest is in helping any interested party figure out how they can contribute to overcoming this shortage. In short, the government should force ventilator makers to make their designs open-source.
Open-source, does not necessarily mean free. It simply means that, in the software industry, the code base is publicly available for anyone to use. That use may incur licensing fees.
Such a licensing program could be put in place now where anyone that uses an open-source design to manufacture equipment will have to pay the company that supplied the design a user fee, like what InVision received after 9/11.
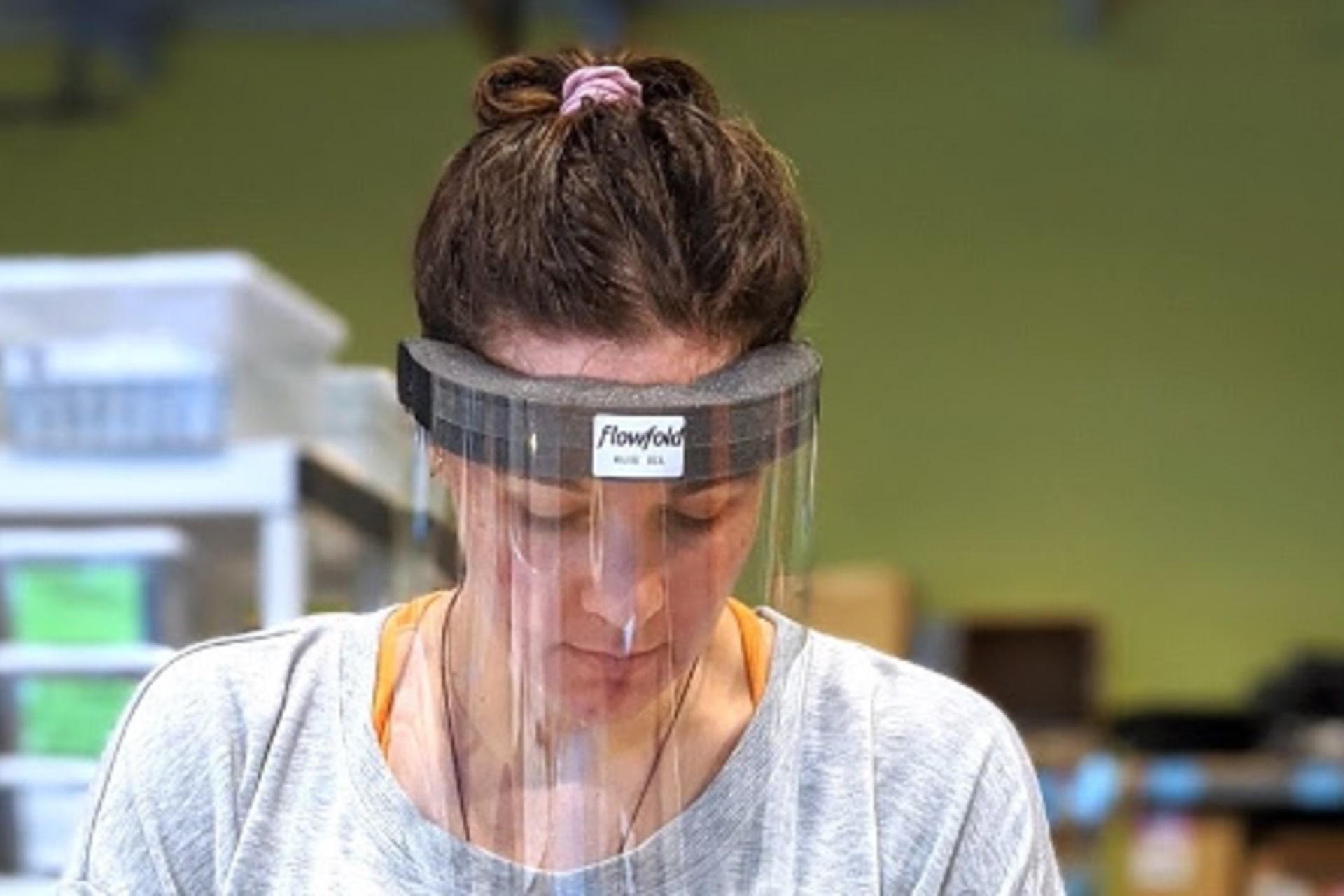
While General Motors has struggled to convert its production facilities to produce respirators, smaller companies have been able to pivot rapidly. In Maine, where I live, Flowfold, a maker of outdoor and travel equipment, has begun to produce face shields to protect hospital workers. STARC Systems, another local company that builds containment walls, is now producing “temporary modular wall systems” to create isolation rooms that can rapidly expand capacity to isolate sick patients.
Thousands of small producers should be able to produce billions of n95 respirators if the plans for those respirators are made publicly available. While ventilators are far more complex devices, those devices consist of many smaller parts. There are thousands of hobbyists with 3-D printers that could be put to work making components. Innovative methods for sterilizing equipment that might not pass FDA approval in normal times but are appropriate shortcuts to take now could be used to address the lack of clean space production facilities.
More complicated components, such as the printed circuit boards that control devices, can be produced by smaller manufacturers scattered throughout the United States. In Maine, there are five such manufacturers, many of whom have struggled but remained open throughout successive rounds of offshoring.
Critics will contend that the complexities of producing ventilators and other medical equipment that is in short supply do not lend themselves to small batch production. If they are right, then there is no harm to releasing the specs and letting small manufacturers take a pass. If they are wrong, then thousands of Americans sitting on the sidelines right now will be able to put their skills to work to get us out of this mess.